
Just a reminder… pay attention to the questions. Here are our general tips one more time:

Just a reminder… pay attention to the questions. Here are our general tips one more time:

Just a reminder… pay attention to the questions. Here are our general tips one more time:

Just a reminder… pay attention to the questions. Here are our general tips one more time:
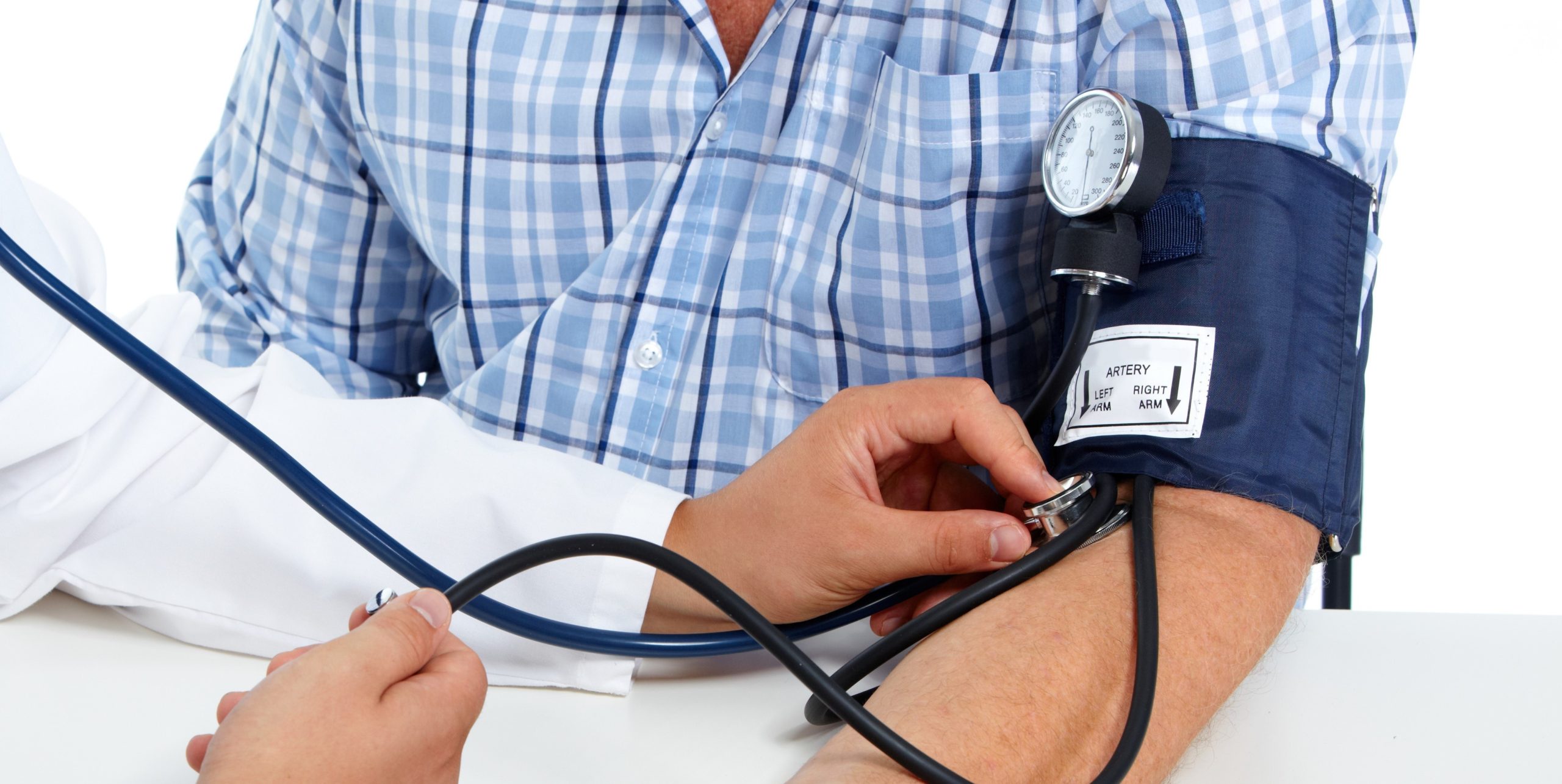
Just a reminder… pay attention to the questions. Here are our general tips one more time:

Just a reminder… pay attention to the questions. Here are our general tips one more time:

Just a reminder… pay attention to the questions. Here are our general tips one more time:
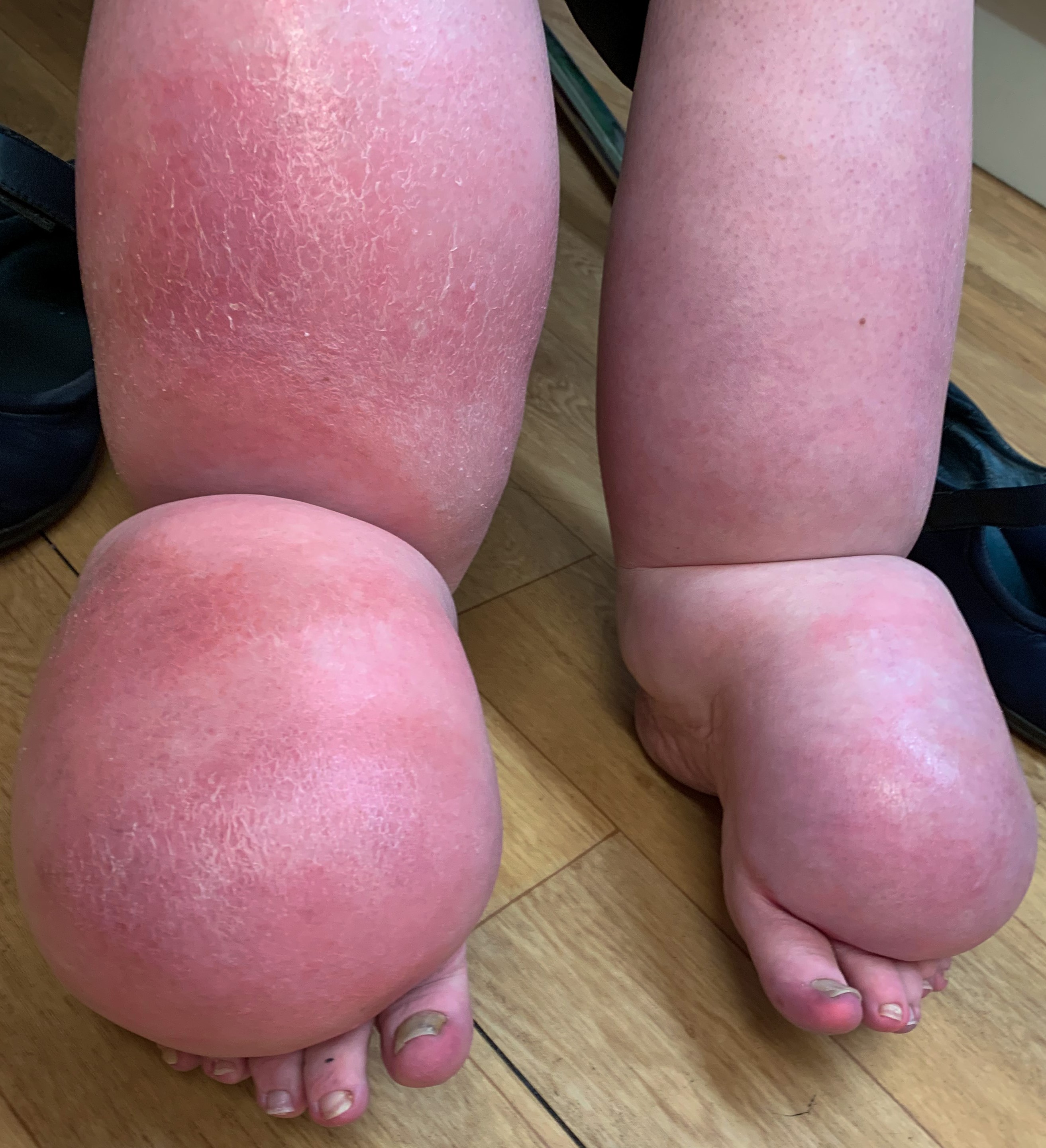
Just a reminder… pay attention to the questions. Here are our general tips one more time:
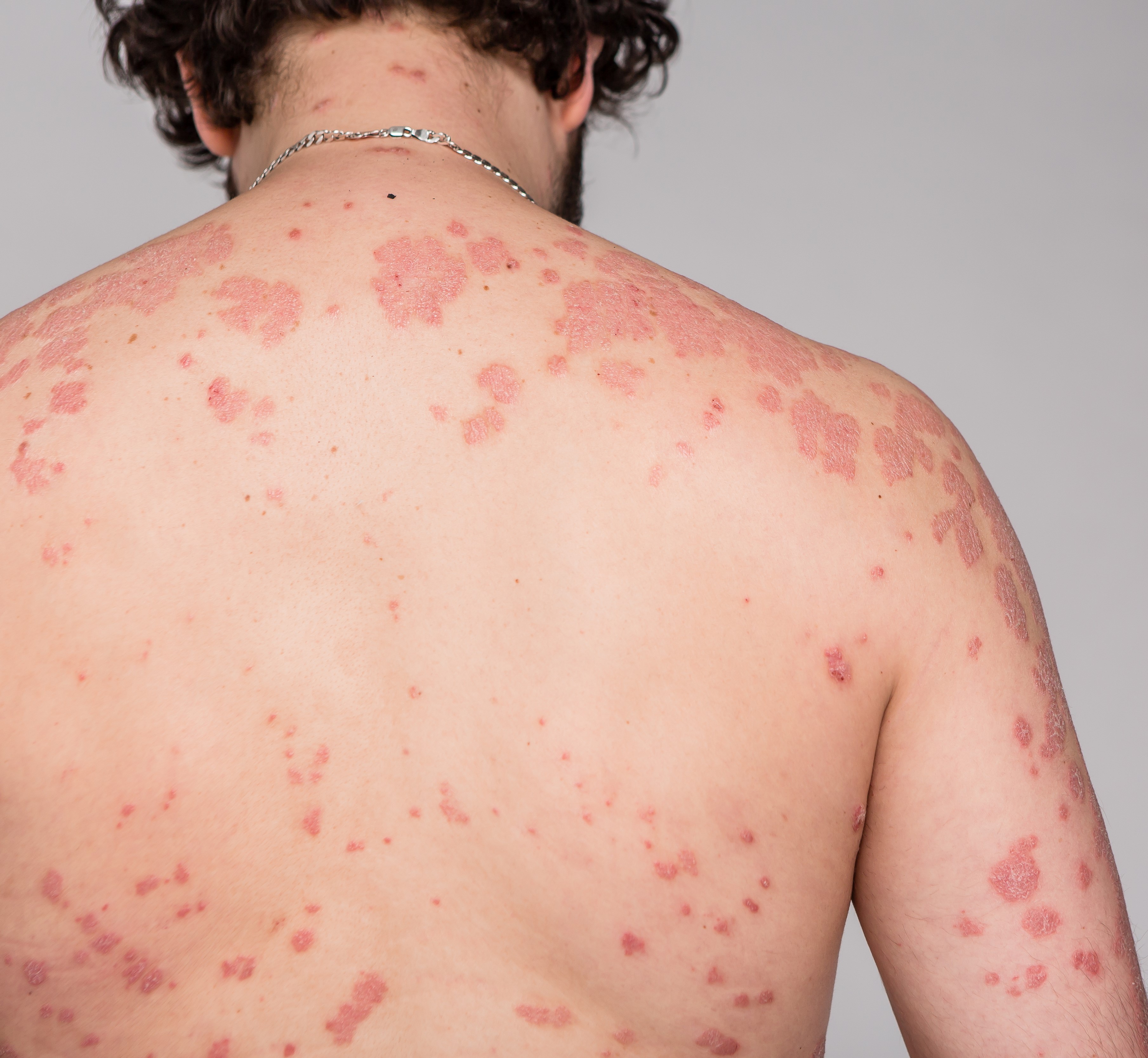
Just a reminder… pay attention to the questions. Here are our general tips one more time:

Just a reminder… pay attention to the questions. Here are our general tips one more time: